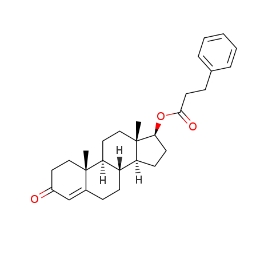
Canonical SMILES:
CC12CCC3C(C1CCC2OC(=O)CCC4=CC=CC=C4)CCC5=CC(=O)CCC35C
Isomeric SMILES:
C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2OC(=O)CCC4=CC=CC=C4)CCC5=CC(=O)CC[C@]35C
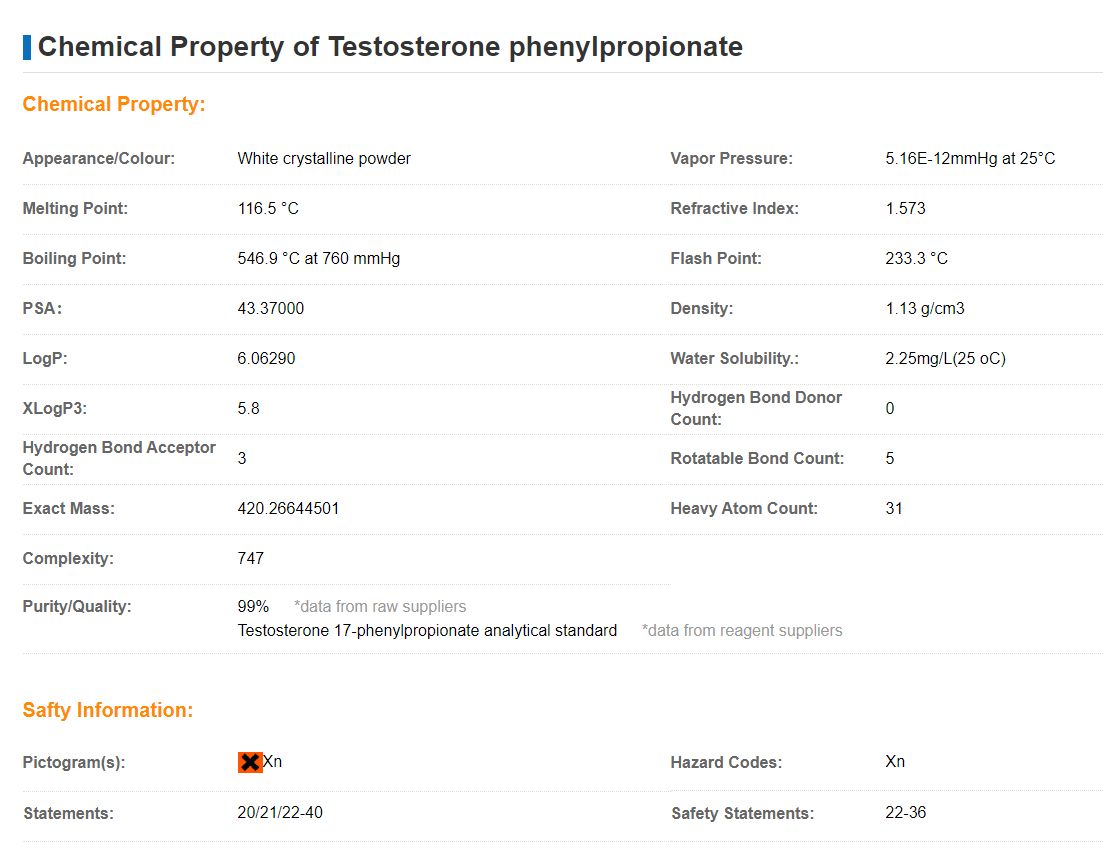
Description
Testosterone phenylpropionate (BAN; TPP) (brand name Testolent), or testosterone phenpropionate, also known as testosterone hydrocinnamate, is a synthetic anabolic-androgenic steroid (AAS) and an androgen ester – specifically, the C17β phenylpropionate ester of testosterone – which was formerly marketed in Romania. It was first synthesized in 1951 and was first described in the literature by 1953. The medication was an ingredient of several isolated AAS commercial products, but was never widely used. Testosterone phenylpropionate was also notably a component of Sustanon and Omnadren, as well as of Estandron Prolongatum, Lynandron Prolongatum, and Mixogen. TPP was previously available in Great Britain.
Biological Functions
Testosterone phenylpropionate is a synthetic anabolic-androgenic steroid (AAS) and an androgen ester. It was first reported in the scientific literature in 1955 and was an ingredient of several isolated AAS commercial products, but was never widely used. Testosterone phenylpropionate was also notably a component of Sustanon and Omnadren.
Technology Process of Testosterone phenylpropionate
There total 15 articles about Testosterone phenylpropionate which guide to synthetic route it. The literature collected by LookChem mainly comes from the sharing of users and the free literature resources found by Internet computing technology. We keep the original model of the professional version of literature to make it easier and faster for users to retrieve and use. At the same time, we analyze and calculate the most feasible synthesis route with the highest yield for your reference as below:
 Zenuo Biotech
Zenuo Biotech