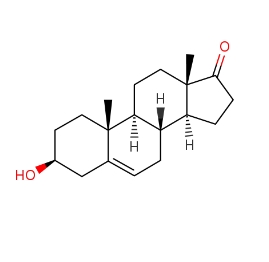
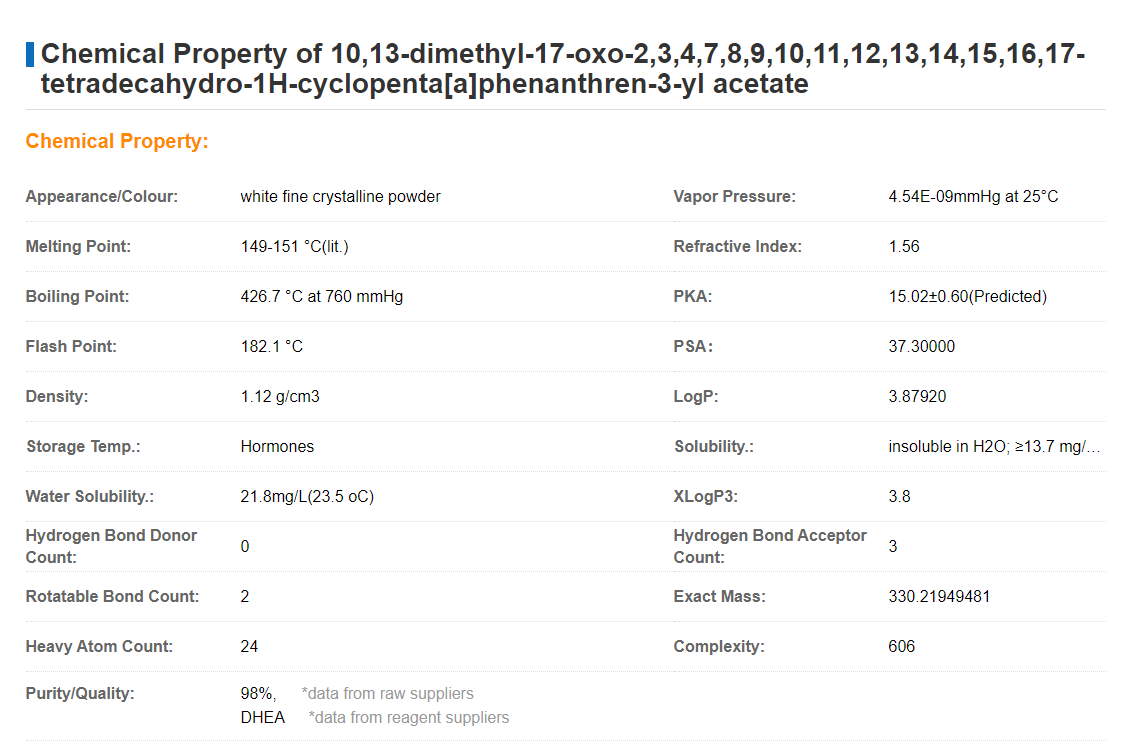
Synonyms:
Spectrum_001089;Spectrum2_001130;Spectrum3_001280;Spectrum4_000244;Spectrum5_000703;BSPBio_002880;KBioGR_000648;
KBioSS_001569;SPECTRUM270029;SCHEMBL370342;SPBio_000999;CHEMBL3039094;KBio2_001569;KBio2_004137;KBio2_006705;
KBio3_002380;Dehydro Epiandrosterone 3-Acetate;HMS1922F18;Pharmakon1600-00270029;CCG-40086;NSC755876;NSC-755876;
NCGC00178411-01;SBI-0207089.P001;AB01563185_01;AA-504/07224052;10,13-dimethyl-17-oxo-2,3,4,7,8,9,10,11,12,13,14,15,16,
17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate
Canonical SMILES:
CC(=O)OC1CCC2(C3CCC4(C(C3CC=C2C1)CCC4=O)C)C
Isomeric SMILES:
CC(=O)O[C@H]1CC[C@@]2(C3CC[C@]4(C(C3CC=C2C1)CCC4=O)C)C
Bone Loss Prevention
Subsequent trials have examined prasterone as a treatment to limit bone loss in women with SLE.
Studies have shown that prasterone can increase bone mineral density in patients who have been taking glucocorticoids for six months or longer, compared to placebo.
Investigation for Systemic Lupus Erythematosus (SLE)
Prasterone, a synthetic dehydroepiandrosterone product, has been investigated for use in women with SLE who are taking glucocorticoids. Initial trials focused on improving disease activity and symptoms in women with mild to moderate SLE, but the FDA did not approve prasterone's labeling for these indications.
Potential Drug Interactions
Prasterone may interact with 5-alpha reductase inhibitors and exhibit additive or antagonistic effects with androgens, estrogens, oral contraceptives, and progestins.
Dosage
In clinical trials, oral prasterone dosages of 100鈥?200 mg/day were administered, resulting in supraphysiological hormone levels.
Intravaginal Use
Intravaginal prasterone, marketed as Intrarosa庐, is approved for the treatment of vulvar and vaginal atrophy (VVA) in postmenopausal women with moderate to severe symptoms.
Clinical trials have demonstrated that intravaginal prasterone significantly improves signs and symptoms of VVA, including dyspareunia, compared to placebo.
Prasterone is generally well-tolerated, with the most common adverse event being application site discharge.
Serum concentrations of estrogenic and androgenic metabolites of DHEA increased during treatment with intravaginal prasterone but remained within normal postmenopausal ranges.
 Zenuo Biotech
Zenuo Biotech